With ongoing testing and a growing body of clinical evidence, remdesivir Gilead is considered a promising treatment for 2019 coronavirus disease (COVID-19). But, according to researchers, in order to develop effective treatment methods, it is necessary to answer several key questions regarding the mechanics of the action of the virus.
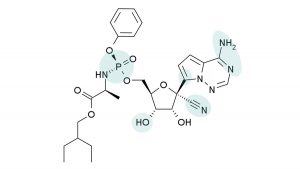 Remdesivir is a nucleotide analog with a broad spectrum of antiviral activity, according to Gilead Sciences Inc. Early studies showed in vitro and in vivo activity in animal models against MERS and severe acute respiratory syndrome, which are closely related to COVID-19.
Remdesivir is a nucleotide analog with a broad spectrum of antiviral activity, according to Gilead Sciences Inc. Early studies showed in vitro and in vivo activity in animal models against MERS and severe acute respiratory syndrome, which are closely related to COVID-19.
According to a review article published in Antimicrobial Agents and Chemotherapy, the drug was also recently tested in a nonhuman primate model of MERS-Coronavirus (CoV) infection. In that research, prophylactic treatment 24 hours prior to inoculation prevented the virus from causing clinical disease and inhibited viral replication in lung tissues. 1 It has also undergone safety testing in clinical trials for Ebola, which the authors noted would reduce the necessary time for conducted clinical trials for severe acute respiratory syndrome coronavirus (SARS-CoV-2)
 Other therapeutic options include a combination of HIV-1 protease inhibitors lopinavir / ritonavir and interferon beta, which has been shown to be effective in patients with acute respiratory viral infections.
Other therapeutic options include a combination of HIV-1 protease inhibitors lopinavir / ritonavir and interferon beta, which has been shown to be effective in patients with acute respiratory viral infections.
The authors noted that therapeutic agents that target only coronavirus may not be able to reverse highly pathogenic infections, which requires a broader approach.
 Despite this progress, an understanding of how SARS-CoV-2 interacts with the host ACE2 receptor may shed light on how the virus crossed the species barrier between animals and humans.
Despite this progress, an understanding of how SARS-CoV-2 interacts with the host ACE2 receptor may shed light on how the virus crossed the species barrier between animals and humans.
In addition, according to researchers, an accurate determination of which animal the virus is transmitted from is necessary to prevent future epidemics associated with SARS in humans.



